
Teebone is an osteoconductive alloplastic material designed for bone substitution. Its biphasic nature allows a controlled resorption profile similar to human bone. Due to its composition, 75% Hydroxiapatite and 25% Beta tricalcium phosphate, Teebone regenerates new bone through two different stages of activation. The breakdown of B-TCP by the osteoclast will free ions that enhance bone grow, while the hydroxyapatite acts as support for the bone ingrown whiting the graft, being slowly substituted.
Indications

Key Features & Benefits

No Membrane
It is not necessary to use membrane, due to its physical and mechanical properties.

Security
100% synthetic and 100% resorbable

Easy Handling
teebone® is very hydrophilic which confers an excellent cohesivity between the particles.

Vascularisation
teebone® induces a remarkable vascularisation.

Total Resorption
teebone® is replaced by new vital bone within 6 -24 months.

Radiopaques
Allows the perfect monitorization of osteointegration.
Device Description
teebone® BCP is a synthetic macroporous ceramic, containing hydroxyapatite and beta tricalcium phosphate, that does not incorporate a medical substance, nor tissues or blood products. The porous synthetic ceramic, containing beta tricalcium phosphate has been widely studied in the market and is not new. teebone® BCP is a sterile, single use, product that is gamma ray radiated using 25kGy. The macroporous ceramic allows an excellent osseointegration, as well as a total vascularization of the implant due to the interconnected porosity. teebone® BCP is designed for the filling of bone voids or defects of the skeletal system, replacing the bone structure gap. Due to the interconnected porosity of teebone® BCP, the implant can be total vascularized which allows an excellent osseointegration. New bone formation will occur and slowly replace the porosity of the implant, and the implant itself.
Intended Use
teebone® BCP, is a porous synthetic ceramic, designed for the filling of bone voids or defects of the skeletal system that are not intrinsic to the stability of the bony structure.
Mechanism of Action
According to the size and geometry of the bone defect, the surgeon establishes the appropriate dosage form to be applied. Irregular bone defects can be filled with granules or combinations of granules, blocks and cylinders.
Bone defects with defined geometric shape can be filled with blocks and cylinders.
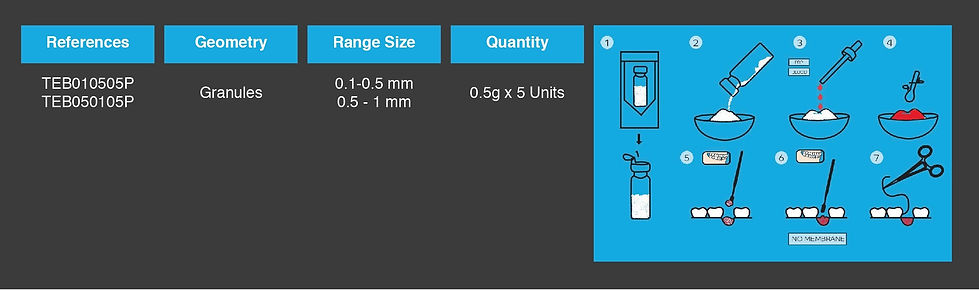
Before implantation, teebone® BCP should be impregnated with patient’s blood or autologous bone marrow, in order to introduce blood cells, growth factors and, in case of bone marrow , osseoprogenitor cells into the graft.
After the impregnation step, the device should be immediately placed into the bone defect, in contact with cancellous tissue. The bone surface must be freshened and slightly bleeding to ensure an appropriate supply of blood cells and growth factors to the entire graft material.
The filling of the bone defect must be completed with a slight impaction and the wound closure must be complete and airtight.
The combination of teebone® BCP with any medical substance during implantation is carried out under the surgeon’s responsibility.
Precautions
As with any surgical procedure, care should be exercised in treating individuals with preexisting conditions that may affect the success of the surgical procedure. This includes individuals with bleeding disorders of any etiology, long-term steroidal therapy, immunosuppressive therapy, or high dosage radiation therapy.
Avoid overfilling the bone defect site or attempt to pressurize the treatment site.
teebone® BCP should not be used at infected sites.
teebone® BCP is not intended for load-bearing uses. It is important to ensure that the area where the teebone® BCP have been implanted be properly secured mechanically with rigid fixations to strengthen the surroundings.
Attempts should not be made to modify the size of the granules or to change their shape. Experienced users may, if required adapt the cylinders or blocks to the defect by careful mechanical reworking prior to implantation, without compromising their function (e.g. pronounced comminution, cutting). Machining the cylinders or block should be performed with them hydrated (e.g. blood or saline solution), and NOT performed in situ to prevent ceramic dust entering the wound bed, which would induce macrophage activity in the form of inflammatory reaction. The dust resulting from this procedure must be carefully removed before inserting the implant.
teebone® BCP must be properly secured to prevent potential migration and should only be used in surgical procedures where bone grafts substitutes are adequately contained.
Composition
teebone® BCP consists of 75% of hydroxyapatite and 25% of β-TCP and comply with ISO 13779-1:2008 – Implants for surgery – Hydroxyapatite – Part 1: Ceramic hydroxyapatite.
Table – teebone® BCP composition.
Compound
Synonyms
Formula
CAS Number
Supplier
Beta-Tricalcium Phosphate
Hydroxyapatite
Tricalcium diphosphate
Calcium hydroxyapatite
β-Ca3(PO4)2
Ca5(PO4)3(OH)
7758-87-4
12167-74-7
Himed
Himed
Control of Raw Materials
All lots of raw materials used in the production of teebone® BCP are fully traceable. The control of raw material is performed according to the following processes:
-
Incoming Inspection
-
All received raw materials and production materials are identified inspected and labeled by qualified personnel according to applicable procedures.
-
The CoC and/or CoA and/or MDS documents, which are obligatory provided by the suppliers are verified and properly maintained.
-
The incoming inspection results are documented, and the records are filed as quality records.
-
The materials are issued for use in the process only after this inspection and approval.
2.Traceability
-
The Company maintains traceability of all lots of raw materials used.
-
All materials and products in all manufacturing process stages are identified and labeled
-
The identification of the products, together with the data and information collected on manufacturing inspection and testing enable full traceability on the product.
-
The Company maintains traceability of lots of products manufactured.
-
This system ensures full information concerning products in case of non-conformities, complaints, returned products or any other reason.
Sterilization Method
teebone® BCP is sterilized by gamma radiation at Synergy Helath, now part of STERIS, at the Reading Facility site (Marcus Close Reading Berkshire RG30 4EA United Kingdom). The Synergy Health firm is certified by the means of the system ISO 9001 / EN ISO 13485 by the BSI certifying body firm and is registered FDA facility nº 3002807310.
Sterilization Validation
The sterilization method was validated according to EN ISO 11137-1:2006 “Sterilization of health care products – Radiation – Part 1: Requirements for development, validation and routine control of a sterilization process for medical devices”, EN ISO 11137-2:2013 “Sterilization of health care products – Radiation – Part 2 : Establishing the sterilization dose, EN ISO 11737-1:2006 “Sterilization of medical devices – Microbiological methods – Part 1: Determination of a population of microorganisms on products” and EN ISO 11737-2:2009 “Sterilization of medical devices – Microbiological methods – Part 2: Tests of sterility performed in the definition, validation and maintenance of a sterilization process”.
The validation of the sterilization method of teebone® BCP included: maximum dose compatibility test, VDmax method and dose mapping.
Packaging Validation
In the packaging system of granules, the sterile barrier is constituted by the pouch. The sterile barrier of the packaging system of the preform shapes is constituted by the blister.
Biocompatibility
The use of hydroxyapatite and β-Tricalcium phosphate in vivo is well known and has been studied and evaluated in numerous animal and human studies over the years. Furthermore, teebone® BCP has been manufactured and distributed in the European market for 7 years without any adverse event to report up to date. The performance of teebone® BCP in the areas of intended uses, have proven that the material is non-toxic, biocompatible, resorbable and gradually replaced by new bone while in contact with vital bone.
